The Role of Clinical Trials in Advancing Healthcare
Clinical Trials in Advancing Healthcare
Introduction: Why Clinical Trials Matter
Modern medicine would not exist without clinical trials. Every vaccine, every new drug, every surgical technique, and even diagnostic tools we take for granted today are products of rigorous clinical research. Clinical trials are the backbone of medical research, providing the evidence needed to determine whether a treatment is safe, effective, and beneficial for patients.
In a rapidly changing healthcare landscape, clinical trials are more than just experiments—they are pathways to innovation, progress, and better patient care. From life-saving cancer therapies to COVID-19 vaccines, clinical trials shape the very foundation of modern healthcare.
What are Clinical Trials?
Clinical trials are systematic studies conducted on human participants to evaluate medical, surgical, or behavioral interventions. They are designed to answer critical questions: Does this treatment work? Is it safe? Is it better than what already exists?
Unlike preclinical studies in labs or animals, clinical trials focus on real human responses. This makes them essential for translating discoveries from laboratories into treatments that can be used in clinics, hospitals, and communities.
Phases of Clinical Trials
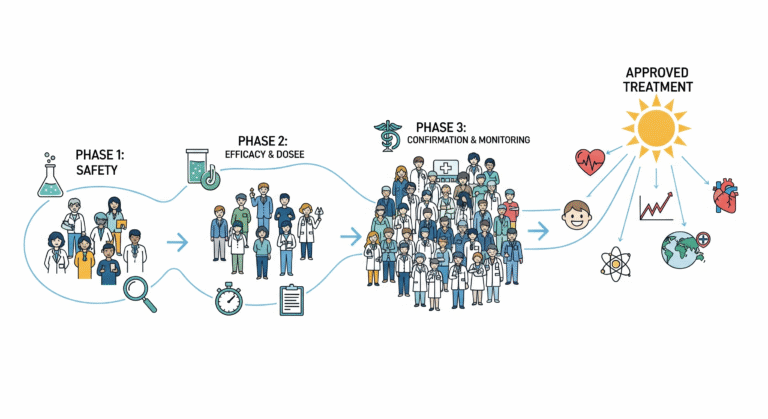
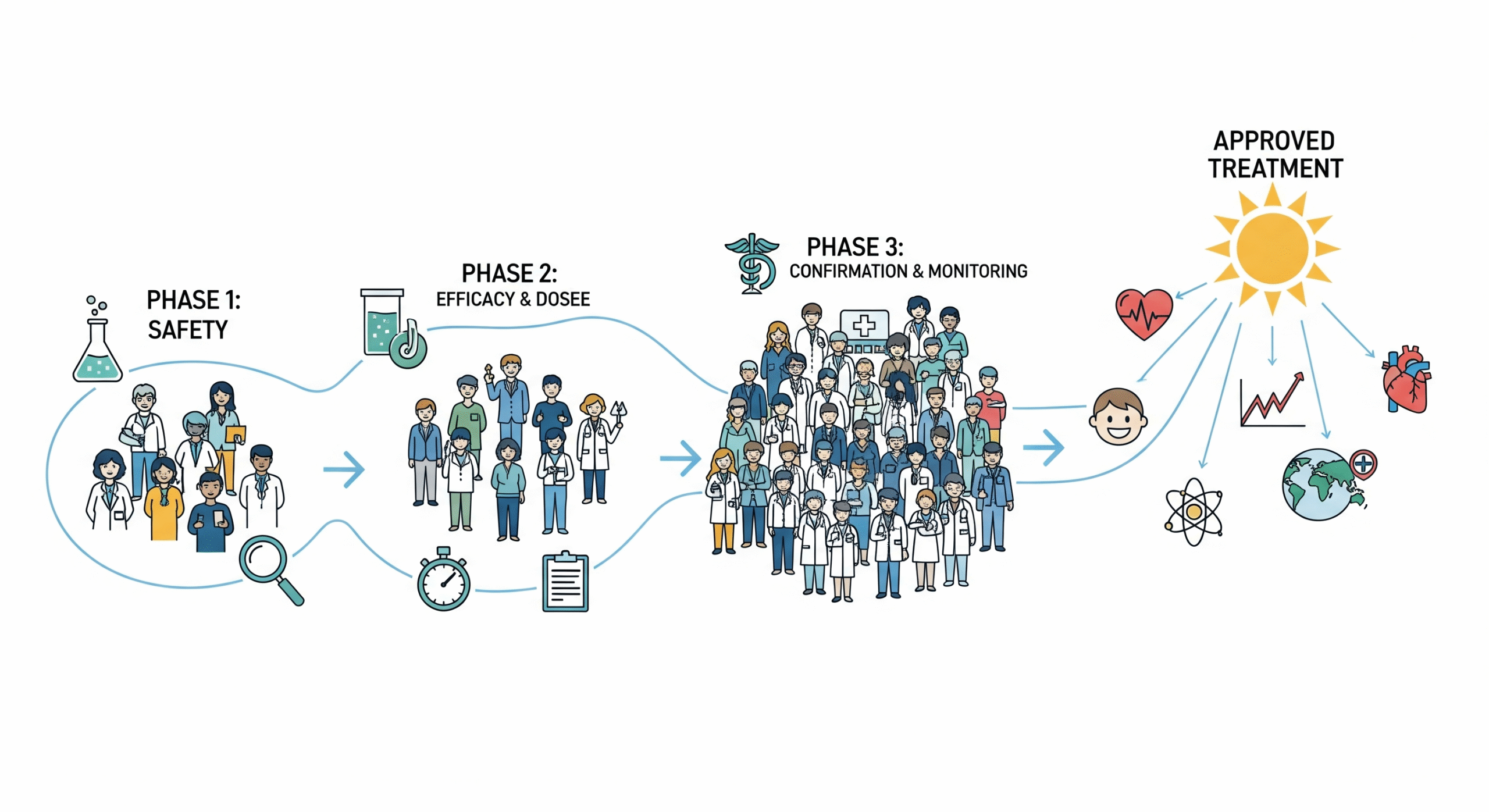
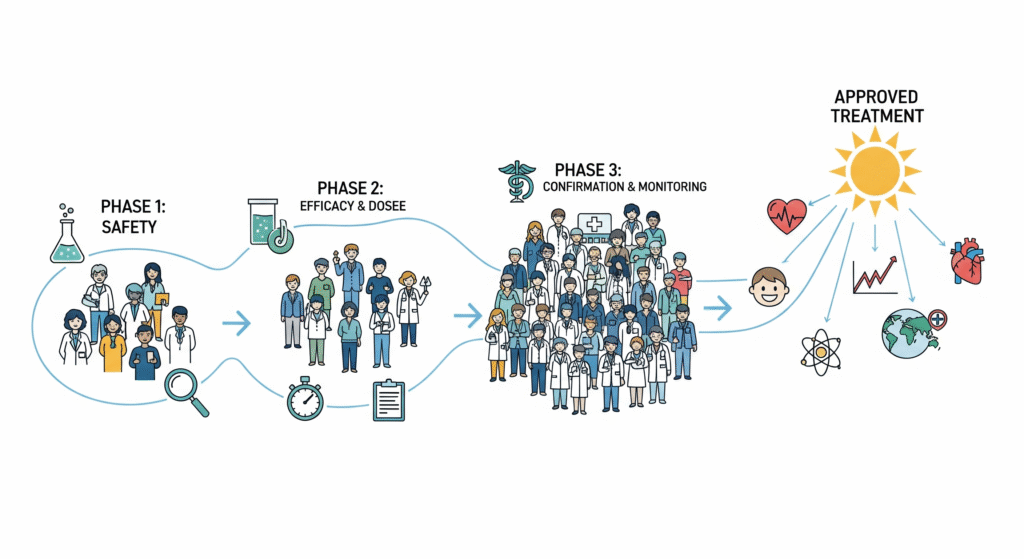
Clinical trials are typically conducted in four major phases, each serving a unique role in the research process.
Phase I trials test a new treatment in a small group to evaluate safety, dosage, and side effects. Phase II expands to a larger group to assess effectiveness and further monitor safety. Phase III trials involve thousands of participants across multiple sites, comparing the new treatment to existing standards of care. If successful, the treatment may receive regulatory approval. Finally, Phase IV trials occur after approval to monitor long-term effectiveness and safety in broader populations.
Each phase builds on the previous one, creating a step-by-step pathway from discovery to widespread adoption in healthcare systems.
Clinical Trials and Evidence-Based Medicine
One of the greatest contributions of clinical trials is their role in evidence-based medicine (EBM). Evidence-based medicine relies on rigorous data rather than tradition, anecdote, or assumption. Clinical trials provide this high-quality evidence, ensuring that treatments are not only scientifically sound but also practical and beneficial in real-world settings.
For example, large-scale randomized controlled trials (RCTs) have established the safety and efficacy of vaccines, blood pressure medications, and cancer therapies. These trials influence guidelines issued by organizations such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC).
How Clinical Trials Advance Healthcare
Clinical trials play a transformative role in advancing healthcare in multiple ways. They bring innovation to patient care, offering new drugs, therapies, and technologies. They establish safety standards, ensuring that treatments do not cause more harm than good. They also improve existing treatments, comparing new interventions with traditional methods to refine clinical practice.
Perhaps most importantly, clinical trials empower patients by giving them access to experimental treatments before they are widely available. This is particularly crucial in fields like oncology, where new therapies can make the difference between life and death.
Clinical trials also influence public health policies by providing data that guide vaccination campaigns, screening programs, and preventive care strategies. Without this research, governments and institutions would be left making decisions in the dark.
Landmark Clinical Trials that Changed Medicine
Throughout history, clinical trials have revolutionized healthcare. The Salk polio vaccine trial in the 1950s protected millions of children from paralysis, making it one of the largest and most successful trials ever conducted. The Women’s Health Initiative in the 1990s reshaped policies on hormone replacement therapy, heart disease, and osteoporosis. More recently, the rapid clinical testing of COVID-19 vaccines demonstrated how rigorous trials could be accelerated without compromising safety, saving millions of lives worldwide.
These landmark examples highlight how clinical trials do more than test treatments—they reshape healthcare systems and improve population health globally.
Challenges in Clinical Trials
Despite their importance, clinical trials face many challenges. One of the biggest barriers is recruitment—finding enough participants who meet the criteria can delay or even derail studies. Costs are another concern, as large-scale trials require millions of dollars in funding.
Ethical concerns also play a major role. Informed consent, patient safety, and transparency must be prioritized to avoid exploitation or harm. There are also representation issues, as many trials historically underrepresented women, children, and minority groups, leading to gaps in understanding how treatments affect diverse populations.
Addressing these challenges requires international cooperation, ethical rigor, and innovation in trial design.
The Role of Technology in Clinical Trials
Modern technology is transforming the way clinical trials are conducted. Artificial intelligence (AI) is being used to identify potential participants, analyze massive datasets, and even predict treatment outcomes. Big data analytics allow researchers to evaluate trial results more efficiently. Wearable health devices provide real-time monitoring of patients outside hospitals, expanding the scope of what can be measured.
Decentralized or “virtual” trials, accelerated by the COVID-19 pandemic, allow participants to join from their homes, making research more inclusive and efficient. These technological advances are reducing costs, speeding up research, and ensuring that trials are more representative of real-world conditions.
Clinical Trials and Global Health
Beyond individual patient care, clinical trials play a critical role in global health. They provide the evidence that shapes vaccination strategies, guides international health policies, and combats diseases like malaria, tuberculosis, and HIV/AIDS.
Global collaboration is essential, as diseases do not respect borders. International clinical trials allow treatments to be tested across different populations, ensuring effectiveness worldwide. They also address health inequalities by bringing advanced research opportunities to low- and middle-income countries.
The Future of Clinical Trials in Healthcare
The future of clinical trials lies in personalized medicine, digital innovation, and global cooperation. Precision medicine will use genetic information to design treatments tailored to individuals, making trials more targeted. Virtual trials will expand participation and reduce barriers. Global partnerships will ensure that research benefits all populations, not just those in high-income countries.
As healthcare becomes more complex, clinical trials will remain the gold standard for testing safety and effectiveness. Strengthening research infrastructure, embracing technology, and ensuring ethical standards will be key to maximizing their impact.
Conclusion: Clinical Trials as Pillars of Progress
Clinical trials are not just a step in the scientific process—they are the pillars of progress in healthcare. They ensure that treatments are safe, effective, and accessible. They bridge the gap between research and patient care, shaping medical guidelines and influencing global health policies.
From eradicating diseases to developing innovative treatments, clinical trials have transformed medicine and will continue to shape the future of healthcare. By investing in clinical research, societies not only advance science but also safeguard the health of current and future generations.
For anyone interested in the future of medicine, understanding the role of clinical trials is essential. They are, and always will be, the foundation upon which modern healthcare stands.
Other related articles at Medical Research and Analysis